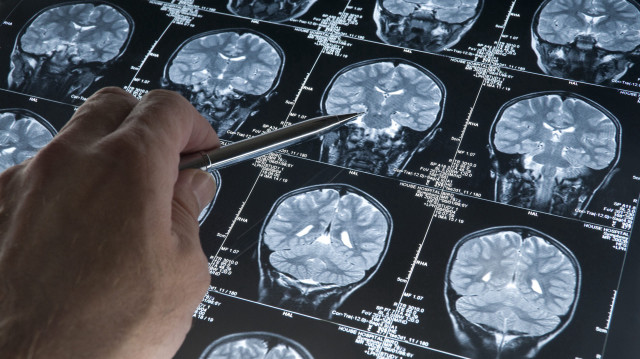
Donanemab has been found to slow progress of Alzheimer's by clearing proteins in brain
Australia's drug and therapeutic regulatory agency has approved the first drug for treatment of Alzheimer's disease, public broadcaster ABC reported on Thursday.
The Therapeutic Goods Administration (TGA) approved the drug, Donanemab, which has been found to slow the progress of Alzheimer's by clearing proteins in the brain.
The drug is meant for those with mild cognitive impairment or mild dementia due to Alzheimer's, which is the most common type of dementia. It is the leading cause of death for Australian women and the second most common for men.
The drug is given as an intravenous infusion through the arm every four weeks for a maximum of 18 months.
Clinical trials have shown it can help in the early stages of Alzheimer's disease.
It is, however, not a cure and does not stop cognitive decline. But it can slow the disease and allow people to have a better quality of life for longer.
The drug, which is already approved in the UK and US, is not listed on the Pharmaceutical Benefits Scheme at this stage, meaning it is not subsidized by the government and will cost anywhere between $40,000 and $80,000 a year to administer.
Health experts, however, caution for side effects such as brain swelling and bleeding, calling for a close monitoring.
*Writing by Aamir Latif